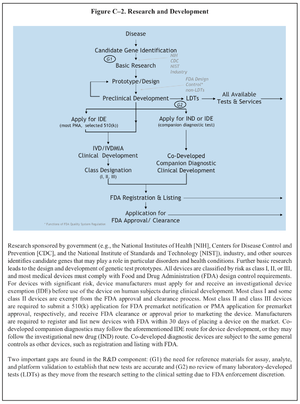
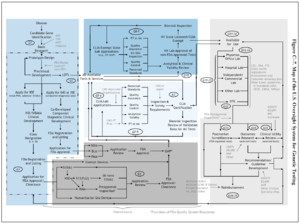
Diagnostic Kits/SACGHS U.S. System of Oversight of Genetic Testing
Jump to navigation
Jump to search
Report of the Secretary’s Advisory Committee on Genetics, Health, and Society, U.S. System of Oversight of Genetic Testing: A Response to the Charge of the Secretary of Health and Human Services, April 2008. Available at: http://oba.od.nih.gov/oba/SACGHS/reports/SACGHS_oversight_report.pdf [Accessed July 8, 2009].
Map of US Oversight for Genetic Testing