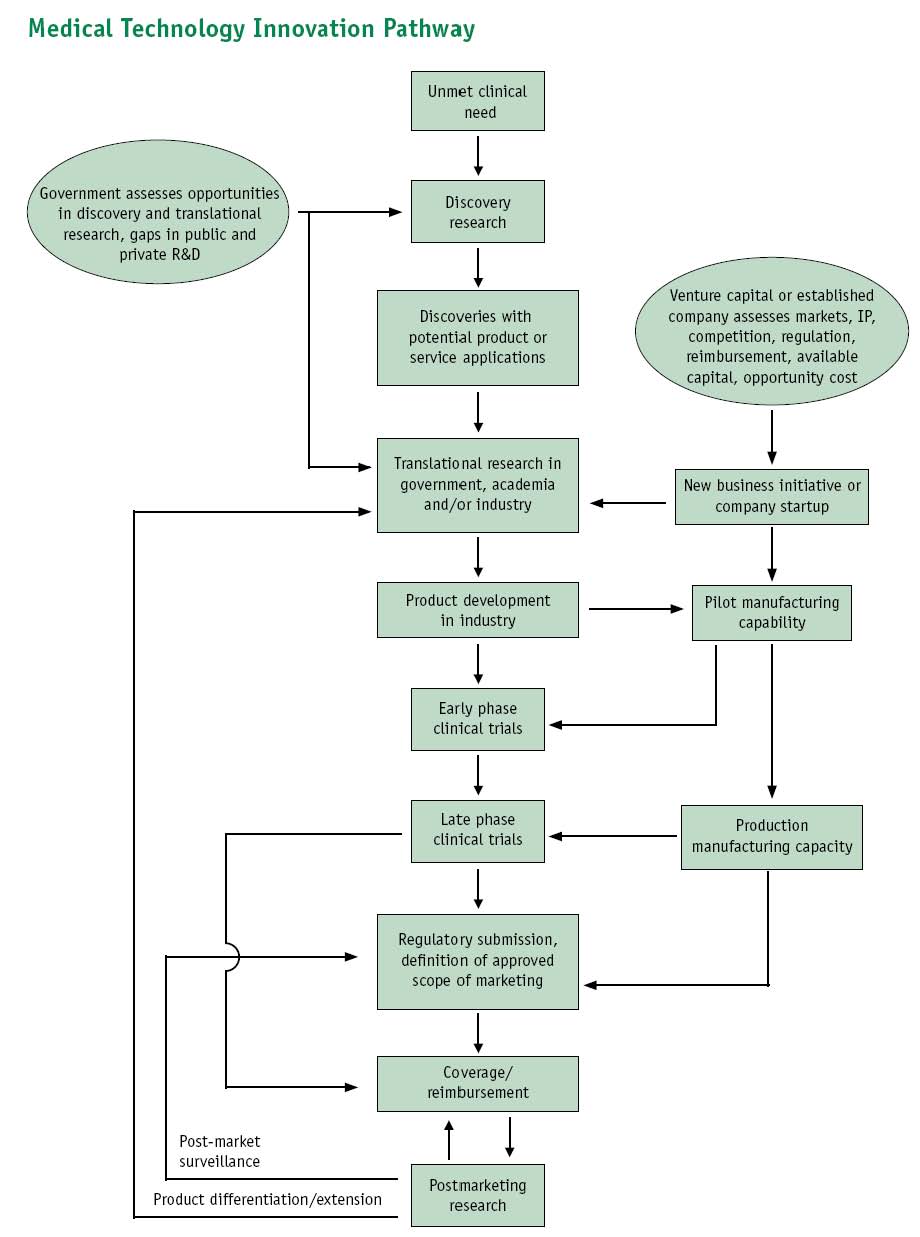
Diagnostic Kits/Give an overall picture of the Kits' sector
Many of these questions can be answered from data in the The Value of Diagnostics report (caution: sponsored by AdvaMed), starting with the Profile of the Diagnostics Industry section.
background: To quantify the Diagnostic Kit Market, we must start with a working definition. When we say Diagnostic Kit, we mean the In-Vitro Diagnostics (IVD), and so we are actually interested in the IVD Market, which encompasses products that produce clinical data from a sample of tissue taken out of a patient (note that other diagnostic products such as medical imagers and in-vivo diagnostics are not included).
IVDs can be categorized based on the location of testing. The vast majority of routine tests are performed in-house hospital labs or in reference labs. These tests may be supplied as a complete kit to the testing laboratory or may be developed in-house with Analyte Specific Reagents (ASRs). ASR-based diagnostics are interesting because the ASRs are sold alone, without specific testing procedures, instructions, or supporting materials. Instead of purchasing a complete kit, labs (which must be CLIA high-complexity certified) purchase just the ASR and develop their own tests around it. Lastly, some kits are available directly to consumers over-the-counter. Of these, some can be operated in the home, such as pregnancy tests and blood glucose tests, while others require the user to ship a sample to a remote reference lab. DNA Direct is a company that operates in this space, providing gene-based OTC diagnostic kits that are evaluated in a remote lab.
- Priorities for Personalized Medicine, prepared by your Council of Advisors on Science and Technology (PCAST). Available at: http://www.ostp.gov/galleries/PCAST/pcast_report_v2.pdf
- "PCAST hopes that this report in its entirety helps lay a foundation for realizing important health care benefits from genomics-based molecular diagnostics, while providing a balanced assessment of the promise and current limitations of personalized medicine more broadly."
- Intellectual Property Section page 21
- "The ability to obtain strong intellectual property protection through patents has been, and will continue to be, essential for pharmaceutical and biotechnology companies to make the large, high-risk R&D investments required to develop novel medical products, including genomics-based molecular diagnostics."
How was this field born and how is it evolving?
What are the main business models?
Regulation
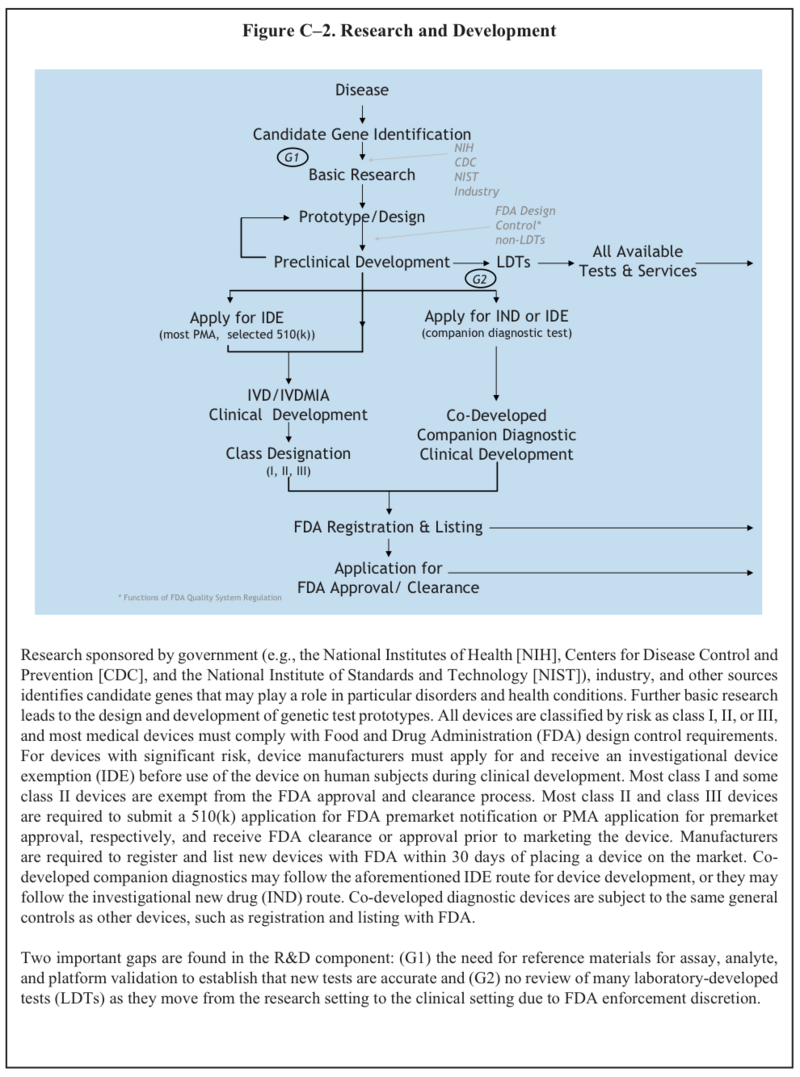
U.S. System of Oversight of Genetic Testing: A Response to the Charge of the Secretary of Health and Human Services, Report of the Secretary’s Advisory Committee on Genetics, Health, and Society, April 2008. pg.242
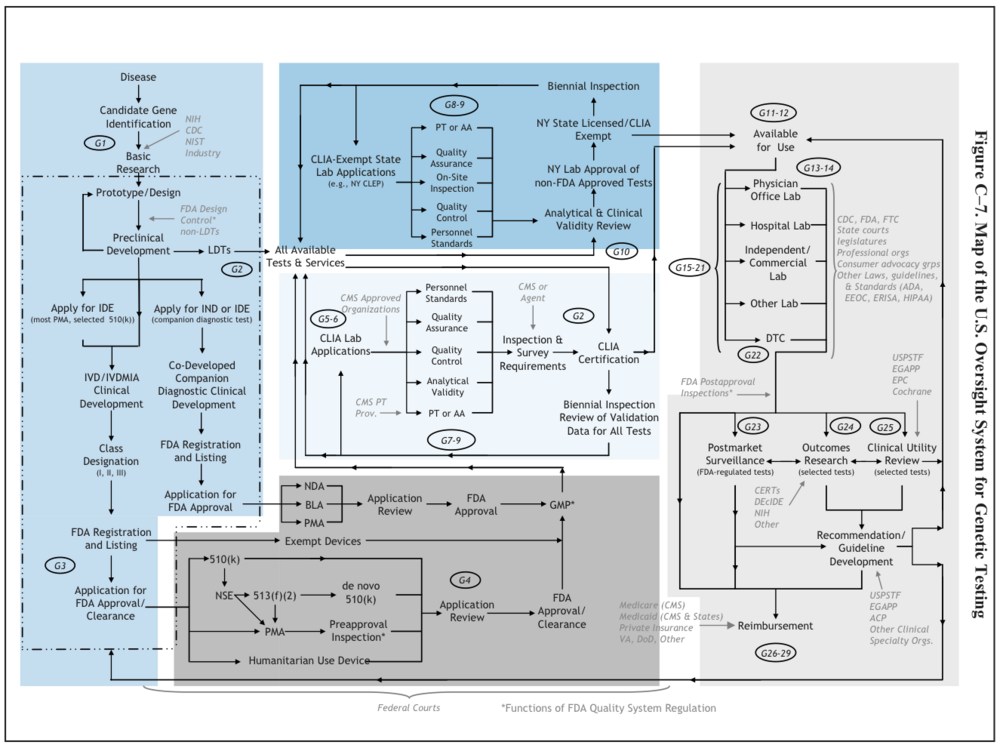
What are the innovation dynamics in this field? (inputs/outputs, timing of innovation/ disruptive or incremental innovation?)
How does knowledge flow in this field?
- Access to Bio-Knowledge: From Gene Patents to Biomedical Materials
- "Recent empirical studies, however, indicate that access to materials is a much more serious problem than patents are for basic biomedical researchers, and access to materials is also a critical problem for producers of biomedical end products like biopharmaceuticals." (quoting the abstract)
- Specific Legislative Efforts
- Genomic Research and Diagnostic Accessibility Act of 2002 (proposed)
- The Genomic Research and Accessibility Act of 2007 (proposed)
- Definition of Biological Knowledge
- Yochai Benkler’s framework for knowledge classification (page 26)
- information
- human knowledge,
- information-embedded tools
- information-embedded goods
- Patent information protects: usually "sequence and function of a given gene" which does not prevent an access problem to basic research (page 28)
- The access problem is in "information-embedded tools"
- See Zhen Lei, Rakhi Juneja & Brian D. Wright, Patents Versus Patenting: Implications of Intellectual Property Protection for Biological Research, 27 NATURE BIOTECHNOLOGY 36, 37 (2009) (a survey of ninety-three US agricultural biology faculty)
- barriers found:
- not patents
- material transfer agreements (MTAs)
- use has increased
- delay has increased: "Thirty-four faculty (42%) experienced a total of ninety-seven delays in research, with an average delay of 8.7 months" (page 28 of [1])
- barriers found:
- John P. Walsh, Wesley M. Cohen & Charlene Cho, Where Excludability Matters: Material Versus Intellectual Property in Academic Biomedical Research, 36 RES. POL’Y 1184, 1191 (2007)
- Of the academics surveyed, "about 75% had made at least one request for materials or data in the past two years, but 18% of requests to academics and 33% of requests to industry went unfulfilled (page 29 of [2]
- non-compliance with MTA requests is an important barrier
- This access problem is governed by social norms
- Adhering to this norm increases the transaction cost of sharing
- How can this cost be reduced? "This cost may be reduced both through initiatives to streamline the contracts covering transfers and through increased use of material depositories." (page 34 of [3])
- Possible Solution: Science Commons Biological Materials Transfer Project
- See Zhen Lei, Rakhi Juneja & Brian D. Wright, Patents Versus Patenting: Implications of Intellectual Property Protection for Biological Research, 27 NATURE BIOTECHNOLOGY 36, 37 (2009) (a survey of ninety-three US agricultural biology faculty)
- Yochai Benkler’s framework for knowledge classification (page 26)
Notes on Research Commercialization Pipeline
It looks something like this, where Dx represents the targets of genetic diagnostics:
Samples(MTAs?), Narratives -> Basic Research -> Dx genes & biomarkers, Narratives -> TTOs (patents) -> Commercialization (licenses) -> Trials / Peer Review -> Marketing
Questions / notes:
- How are human tissue samples controlled during the basic research stage?
- Are putative Dx genes and biomarkers always patented by the TTO before publication? Or are some described in literature before IP?
- The value of a research output a TTO protects is often unclear, hence the TTO is willing to negotiate for an exclusive license to make the protected technology as desirable from a commercialization perspective as possible
- Often small start-ups obtain exclusive licenses from the TTOs. Are they often then acquired by large corporations? Is it easier for large companies to purchase small ones w/ exclusive licenses rather than negotiating w/ TTOs to begin with?
Image of Pipeline diagram provided in: Priorities for Personalized Medicine, prepared by your Council of Advisors on Science and Technology (PCAST). Available at: http://www.ostp.gov/galleries/PCAST/pcast_report_v2.pdf
Is this field replicating models from other fields?
How many companies?
Diagnostic Kit Manufacturers
Diagnostic laboratories
Hospital Labs
References labs
"The major national reference labs including Quest, LabCorp, Specialty/Ameripath, Mayo and others account for at least 60% of the market for esoteric test services. The remaining 40% is shared by a group of some 3000 small, local market laboratories. The major reference labs have built a comprehensive menu of specialized test services and continue to expand their offerings via collaborations with leading medical research centers. They offer a huge presence in the market and manage distribution networks that touch just about every medical specialty. Thus many CLIA-registered company sponsored test services avail themselves of the marketing resources offered by the national reference labs."
- CLIA labs offering ASR-based diagnostics:
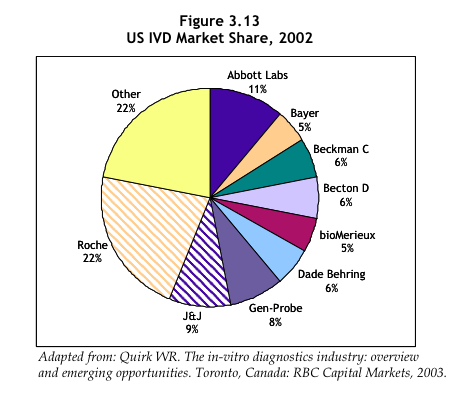 "In 2002, nine companies accounted for 78% of the US diagnostics market" - The Value of Diagnostics pg. 53, referencing Quirk WR. The in-vitro diagnostics industry: overview and emerging opportunities. Toronto, Canada: RBC Capital Markets, 2003. (ref kalorama)
"In 2002, nine companies accounted for 78% of the US diagnostics market" - The Value of Diagnostics pg. 53, referencing Quirk WR. The in-vitro diagnostics industry: overview and emerging opportunities. Toronto, Canada: RBC Capital Markets, 2003. (ref kalorama)
- Roche: 22%
- Abbott Labs: 11%
- J&J: 9%
- Gen-Probe: 8%
- Beckman C: 6%
- Becton D: 6%
- Dade Behring: 6%
- Bayer: 5%
- BioMerieux: 5%
- other: 22%