Diagnostic Kits/SACGHS U.S. System of Oversight of Genetic Testing: Difference between revisions
Jump to navigation
Jump to search
AClearwater (talk | contribs) (New page: Report of the Secretary’s Advisory Committee on Genetics, Health, and Society, U.S. System of Oversight of Genetic Testing: A Response to the Charge of the Secretary of Health and Human ...) |
No edit summary |
||
| Line 1: | Line 1: | ||
Report of the Secretary’s Advisory Committee on Genetics, Health, and Society, U.S. System of Oversight of Genetic Testing: A Response to the Charge of the Secretary of Health and Human Services, April 2008. Available at: http://oba.od.nih.gov/oba/SACGHS/reports/SACGHS_oversight_report.pdf [Accessed July 8, 2009]. | Report of the Secretary’s Advisory Committee on Genetics, Health, and Society, U.S. System of Oversight of Genetic Testing: A Response to the Charge of the Secretary of Health and Human Services, April 2008. Available at: http://oba.od.nih.gov/oba/SACGHS/reports/SACGHS_oversight_report.pdf [Accessed July 8, 2009]. | ||
'''Map of US Oversight for Genetic Testing''' | |||
{| | |||
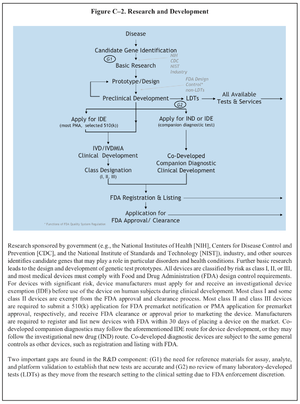
| [[Image:US System of Oversight of Genetic Testing p242 C-4 Research and Development - 2008 SAGCHS.png|thumbnail|The report points out that at "(G1) there is a need for reference materials for assay, analyte, and platform validation to establish that new tests are accurate."]] | |||
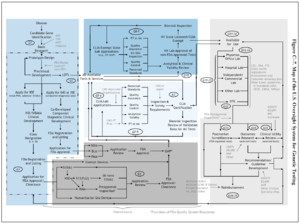
| [[Image:US System of Oversight of Genetic Testing p247 - 2008 SAGCHS.png|thumbnail|This image presents a map of the US regulatory process for diagnositics and implicitly demonstrates the diagnostic development pipeline.]] | |||
|} | |||
Revision as of 10:24, 2 October 2009
Report of the Secretary’s Advisory Committee on Genetics, Health, and Society, U.S. System of Oversight of Genetic Testing: A Response to the Charge of the Secretary of Health and Human Services, April 2008. Available at: http://oba.od.nih.gov/oba/SACGHS/reports/SACGHS_oversight_report.pdf [Accessed July 8, 2009].
Map of US Oversight for Genetic Testing